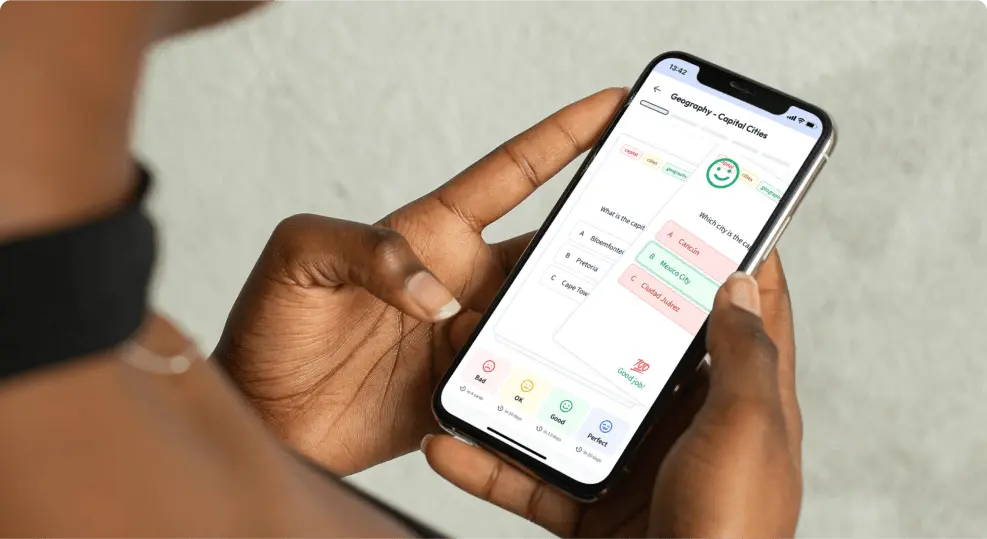
Chemical bonding is very similar to this. Some atoms are much happier by themselves, while some prefer to join up with others. They do this by forming chemical bonds.
Chemical bonding is the attraction between different atoms that enables the formation of molecules or compounds. It occurs thanks to the sharing, transfer, or delocalization of electrons.
- This article is an introduction to the types of bonding in chemistry.
- We will look at why atoms bond.
- We'll explore the three types of chemical bonds.
- We'll then look at factors affecting the strength of bonding.
Why Do Atoms Bond?
At the start of this article, we introduced you to a chemical bond: the attraction between different atoms that enables the formation of molecules or compounds. But why do atoms bond to each other in this way?
Simply put, atoms form bonds in order to become more stable. For the majority of atoms, this means obtaining a full outer shell of electrons. An atom's outer shell of electrons is known as its valence shell; these valence shells typically require eight electrons to fill them up completely. This gives them the electron configuration of the noble gas closest to them in the periodic table. Achieving a full valence shell puts the atom in a lower, more stable energy state, which is known as the octet rule.
The octet rule states that the majority of atoms tend to gain, lose, or share electrons until they have eight electrons in their valence shell. This gives them the configuration of a noble gas.
But to get to this more stable energy state, atoms might need to move some of their electrons around. Some atoms have too many electrons. They find it easiest to get a full valence shell by getting rid of surplus electrons, either by donating them to another species, or by delocalizing them. Other atoms don't have quite enough electrons. They find it easiest to gain extra electrons, either by sharing them or accepting them from another species.
When we say 'easiest', we really mean 'most energetically favorable'. Atoms don't have preferences - they're simply subject to the laws of energy that govern the whole universe.
You should also note that there are some exceptions to the octet rule. For example, the noble gas helium has just two electrons in its outer shell and is perfectly stable. Helium is the noble gas nearest to a handful of elements such as hydrogen and lithium. This means that these elements are also more stable when they have just two outer shell electrons, not the eight that the octet rule predicts. Check out The Octet Rule for more information.
Moving electrons around creates differences in charges, and differences in charges cause attraction or repulsion between atoms. For example, if one atom loses an electron, it forms a positively charged ion. If another atom gains this electron, it forms a negatively charged ion. The two oppositely charged ions will be attracted to each other, forming a bond. But this is just one of the ways of forming a chemical bond. In fact, there are a few different types of bonds that you need to know about.
Types of Chemical Bonds
There are three different types of chemical bonds in chemistry.
These are all formed between different species and have different characteristics. We'll start by exploring the covalent bond.
Covalent Bonds
For some atoms, the simplest way to achieve a filled-up outer shell is by gaining extra electrons. This is typically the case with non-metals, which contain a large number of electrons in their outer shell. But where can they get extra electrons from? Electrons don't just appear out of nowhere! Non-metals get around this in an innovative way: they share their valence electrons with another atom. This is a covalent bond.
A more accurate description of covalent bonding involves atomic orbitals. Covalent bonds form when valence electron orbitals overlap, forming a shared pair of electrons. The atoms are held together by electrostatic attraction between the negative electron pair and the atoms' positive nuclei, and the shared pair of electrons counts towards the valence shell of both bonded atoms. This enables them both to effectively gain an extra electron, bringing them closer to a full outer shell.
 Fig.1-Covalent bonding in fluorine.
Fig.1-Covalent bonding in fluorine.
In the example above, each fluorine atom starts with seven outer shell electrons - they're one short of the eight needed to have a full outer shell. But both fluorine atoms can use one of their electrons to form a shared pair. In this way, both atoms seemingly end up with eight electrons in their outer shell.
There are three forces involved in covalent bonding.
- The repulsion between the two positively charged nuclei.
- The repulsion between the negatively charged electrons.
- The attraction between the positively charged nuclei and the negatively charged electrons.
If the total strength of the attraction is stronger than the total strength of the repulsion, the two atoms will bond.
Multiple Covalent Bonds
For some atoms, such as fluorine, just one covalent bond is enough to give them that magic number of eight valence electrons. But some atoms might have to form multiple covalent bonds, sharing further pairs of electrons. They can either bond with multiple different atoms, or form a double or triple bond with the same atom.
For example, nitrogen needs to form three covalent bonds in order to achieve a full outer shell. It can either form three single covalent bonds, one single and one double covalent bond, or one triple covalent bond.
 Fig.2-Single, double, and triple covalent bonds
Fig.2-Single, double, and triple covalent bonds
Covalent Structures
Some covalent species form discrete molecules, known as simple covalent molecules, made up of just a few atoms joined with covalent bonds. These molecules tend to have low melting and boiling points. But some covalent species form giant macromolecules, made up of an infinite number of atoms. These structures have high melting and boiling points. We saw above how a fluorine molecule is made up of just two fluorine atoms covalently bonded together. Diamond, on the other hand, contains many hundreds of atoms covalently bonded together - carbon atoms, to be precise. Each carbon atom forms four covalent bonds, creating a giant lattice structure that stretches in all directions.
 Fig.3-A representation of the lattice in a diamond
Fig.3-A representation of the lattice in a diamond
Check out Covalent Bonding for a more detailed explanation of covalent bonds. If you want to know more about covalent structures and the properties of covalent bonds, head over to Bonding and Elemental Properties.
Ionic Bonds
Above, we learned how non-metals effectively 'gain' extra electrons by sharing an electron pair with another atom. But bring metal and a non-metal together, and they can do one better - they actually transfer an electron from one species to the other. The metal donates its extra valence electrons, bringing it down to eight in its outer shell. This forms a positive cation. The non-metal gains these donated electrons, bringing the number of electrons up to eight in its outer shell, forming a negative ion, called an anion. In this way, both elements are satisfied. The oppositely charged ions are then attracted to each other by strong electrostatic attraction, forming an ionic bond.
An ionic bond is an electrostatic attraction between oppositely charged ions.
 Fig.4-Ionic bonding between sodium and chlorine
Fig.4-Ionic bonding between sodium and chlorine
Here, sodium has one electron in its outer shell, whilst chlorine has seven. In order to achieve a complete valence shell, sodium needs to lose one electron whilst chlorine needs to gain one. Sodium, therefore, donates its outer shell electron to chlorine, transforming into a cation and an anion respectively. The oppositely charged ions are then attracted to each other by electrostatic attraction, holding them together.
When the loss of an electron leaves an atom with no electrons in its outer shell, we consider the shell below as the valence shell. For example, the sodium cation has no electrons in its outer shell, so we look to the one below - which has eight. Sodium, therefore, satisfies the octet rule. This is why group VIII is often called group 0; for our purposes, they mean the same thing.
Ionic Structures
Ionic structures form giant ionic lattices made up of many oppositely charged ions. They don't form discrete molecules. Each negatively charged ion is ionically bonded to all of the positively charged ions around it, and vice versa. The sheer number of ionic bonds gives ionic lattices high strength, and high melting and boiling points.
 Fig.5-An ionic lattice structure
Fig.5-An ionic lattice structure
Covalent bonding and ionic bonding are actually closely related. They exist on a scale, with completely covalent bonds at one end and completely ionic bonds at the other. Most covalent bonds exist somewhere in the middle. We say that bonds that behave a little like ionic bonds have an ionic 'character'.
Metallic Bonds
Now we know how non-metals and metals bond with each other, and how non-metals bond with themselves or with other non-metals. But how do metals bond? They have the opposite problem to non-metals - they have too many electrons, and the easiest way for them to achieve a full outer shell is by losing their extra electrons. They do this in a special way: by delocalizing their valence shell electrons.
What happens to these electrons? They form something called a sea of delocalization. The sea surrounds the remaining metals centers, which arrange themselves into an array of positive metal ions. The ions are held in place by electrostatic attraction between themselves and the negative electrons. This is known as a metallic bond.
Metallic bonding is a type of chemical bonding found within metals. It consists of the electrostatic attraction between an array of positive metal ions and a sea of delocalized electrons.
It is important to note that the electrons aren't associated with any one metal ion in particular. Instead, they move freely between all of the ions, acting both as a glue and a cushion. This leads to good conductivity in metals.
 Fig.6-Metallic bonding in sodium
Fig.6-Metallic bonding in sodium
We learned earlier that sodium has one electron in its outer shell. When sodium atoms form metallic bonds, each sodium atom loses this outer shell electron to form a positive sodium ion with a charge of +1. The electrons form a sea of delocalization surrounding the sodium ions. The electrostatic attraction between the ions and the electrons is known as a metallic bond.
Metallic Structures
Like ionic structures, metals form giant lattices that contain an infinite number of atoms and stretch in all directions. But unlike ionic structures, they are malleable and ductile, and they usually have slightly lower melting and boiling points.
Bonding and Elemental Properties contains all you need to know about how bonding affects the properties of different structures.
Summarising Types of Bonds
We've made you a handy table to help you compare the three different types of bonding. It summarises all you need to know about covalent, ionic, and metallic bonding.
| Covalent | Ionic | Metallic |
| Description | Shared pair of electrons | Transfer of electrons | Delocalization of electrons |
| Electrostatic forces | Between the shared pair of electrons and the atoms' positive nuclei | Between oppositely charged ions | Between positive metal ions and the sea of delocalized electrons |
| Structures formed | Simple covalent moleculesGiant covalent macromolecules | Giant ionic lattices | Giant metallic lattices |
| Diagram | 
| 
| 
|
The Strength of Chemical Bonds
If you had to guess, which type of bonding would you label as the strongest? It is actually ionic > covalent > metallic bonding. But within each type of bonding, there are certain factors that influence the bond's strength. We'll start by looking at the strength of covalent bonds.
Strength of Covalent Bonds
You'll remember that a covalent bond is a shared pair of valence electrons, thanks to the overlap of electron orbitals. There are a few factors that affect the strength of a covalent bond, and they all have to do with the size of this area of orbital overlap. These include the type of bond and the size of the atom.
- As you move from a single covalent bond to a double or triple covalent bond, the number of overlapping orbitals increases. This increases the strength of the covalent bonding.
- As the size of the atoms increases, the proportional size of the area of orbital overlap decreases. This decreases the strength of the covalent bonding.
- As polarity increases, the strength of the covalent bonding increases. This is because the bond becomes more ionic in character.
Strength of Ionic Bonds
We now know that an ionic bond is an electrostatic attraction between oppositely charged ions. Any factors that affect this electrostatic attraction affect the strength of the ionic bond. These include the charge of the ions and the size of the ions.
- Ions with a higher charge experience stronger electrostatic attraction. This increases the strength of the ionic bonding.
- Ions with a smaller size experience stronger electrostatic attraction. This increases the strength of the ionic bonding.
Visit Ionic Bonding for a deeper exploration of this topic.
Strength of Metallic Bonds
We know that a metallic bond is an electrostatic attraction between an array of positive metal ions and a sea of delocalized electrons. Once again, any factors that affect this electrostatic attraction affect the strength of the metallic bond.
- Metals with more delocalized electrons experience stronger electrostatic attraction, and stronger metallic bonding.
- Metals ions with a higher charge experience stronger electrostatic attraction, and stronger metallic bonding.
- Metal ions with a smaller size experience stronger electrostatic attraction, and stronger metallic bonding.
You can find out more at Metallic Bonding.
Bonding and Intermolecular Forces
It is important to note that bonding is completely different from intermolecular forces. Chemical bonding occurs within a compound or molecule and is very strong. Intermolecular forces occur between molecules and are much weaker. The strongest type of intermolecular force is a hydrogen bond.
Despite its name, it is not a type of chemical bond. In fact, it is ten times weaker than a covalent bond!
Go to Intermolecular Forces to find out more about hydrogen bonds and the other types of intermolecular forces.
Types of Chemical Bonds - Key takeaways
- Chemical bonding is the attraction between different atoms that enables the formation of molecules or compounds. Atoms bond to become more stable according to the octet rule.
- A covalent bond is a shared pair of valence electrons. It typically forms between non-metals.
- An ionic bond is an electrostatic attraction between oppositely charged ions. It typically occurs between metals and non-metals.
- A metallic bond is an electrostatic attraction between an array of positive metal ions and a sea of delocalized electrons. It forms within metals.
- Ionic bonds are the strongest type of chemical bond, followed by covalent bonds and then metallic bonds. Factors affecting the strength of bonding include the size of atoms or ions, and the number of electrons involved in the interaction.